
About our Research
NORSE (New Onset Refractory Status Epilepticus) (and its sub-type FIRES, Febrile Infection-Related Epilepsy Syndrome) remains one of the most challenging acute, devastating neurological disorders.
Currently, the role of immune dysregulation and immune-modifying treatments are of special interest. In this newly-defined space, however, our research priorities remain broad and collaborative, reflecting the complexity and rarity of these syndromes and how much remains unknown.
Etiology
We focus on identifying biomarkers and risk factors that might explain the underlying causes and mechanisms of NORSE/FIRES, especially cryptogenic or c-norse. This includes investigating genetic, immunologic, inflammatory, microbiome-related, infectious, or other potential contributors. Our approach spans both animal models and human studies to better understand pathophysiology.
Read about some of the research into etiology and mechanisms by our Medical & Scientific Advisory Board members and their colleagues below.
Natural History
We encourage the deep phenotyping of NORSE/FIRES patients via detailed clinical and paraclinical data, such as imaging, EEG, cytokine profiles, and treatment responses to explore how these factors influence outcomes and therapeutic effectiveness.
We are at the forefront of expanding the focus of research to include the chronic phase of NORSE/FIRES and their long-term outcomes.
Ongoing Research
-

Cytokine Analysis from NORSE/FIRES Biorepository
For patients in the acute phase, cytokine results from donated bio samples can be reported back promptly which may guide treatment. Yale handles the consent process with the families and covers the cost of shipping.
-
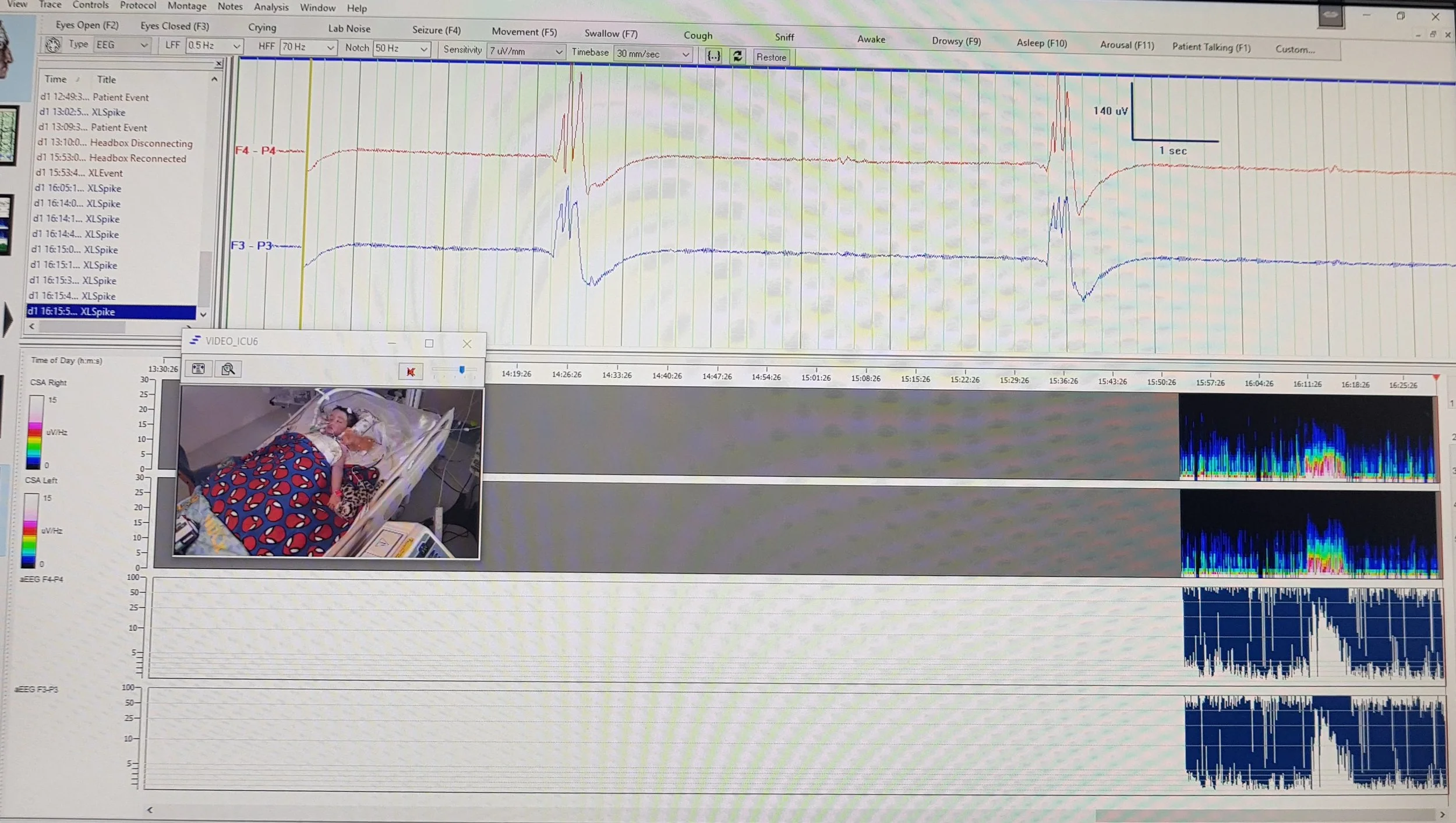
Prospective Observational Study
A prospective, observational study enrolling both adult and pediatric NORSE/FIRES patients. Clinical data and biological samples are collected.
-
Comparing the Effects of Anakinra and Tocilizumab on Outcomes in Patients With NORSE
Randomized PCORI-funded clinical trial over 5 years. Expected patient enrollment: 310. Adult and pediatric patients ages 2 and over. PI: Lawrence Hirsch, MD, (Yale) Planned start - October 2025.
-

Doctor/Family Communication in the ICU
Families of NORSE/FIRES patients have voiced their strong feelings about the good and bad aspects of communicating with medical staff while in the ICU--and researchers have listened. Drs. Krista Eschbach (Children's Hospital - Colorado) and Kristen Sydney Fisher (Baylor/TCH) have initiated a study to formally record and analyze family feedback on this topic. Please take this opportunity to have your views included. Eligible participants in the study are parents and caregivers of patients, surviving or deceased.
NORSE Research Bulletin
Distributed by email to over 300 medical professionals, this newsletter includes upcoming conferences and webinars, grant and research opportunities, and relevant publications.
To subscribe or submit research, funding opportunities, or publications, email nora.norse@gmail.com


